Contents
1. WellPCB
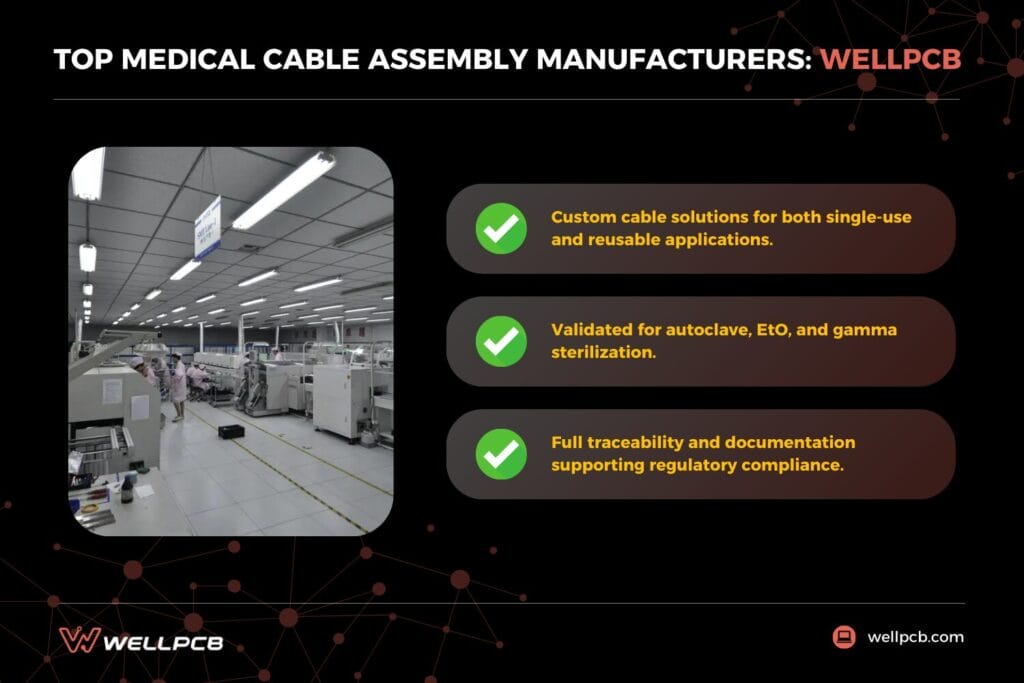
WellPCB shines as a one-stop manufacturing partner for medical device teams that want to move from early design to validated production without managing multiple suppliers. By combining PCB manufacturing, cable assembly and final assembly under one roof, WellPCB shortens timelines and lowers risk for fast-moving medical programs.
This integrated approach allows design feedback to stay closely tied to manufacturing reality. Medical cable assemblies are built using medical-grade materials such as thermoplastic polyurethane (TPU) and thermoplastic elastomer (TPE), with designs validated for autoclave, ethylene oxide (EtO) and gamma sterilization. These capabilities support both reusable and single-use applications while maintaining consistency across connected subassemblies.
Quality and compliance are reinforced through in-house electrical, mechanical and dimensional testing, along with full assembly-level traceability and documentation. Certified to ISO 9001:2015, WellPCB supports prototype builds and scaled production without sacrificing control, making it a strong fit for teams balancing speed, cost and regulatory expectations.
| Pros | Cons |
|---|---|
| ✅ One-stop manufacturing reduces supplier coordination and production risk. | ❌ Limited off-the-shelf options for immediate shipment. |
| ✅ Integrated design feedback improves manufacturability and time-to-market. | |
| ✅ Validated for autoclave, EtO and gamma sterilization. |
2. Proterial Cable America
Proterial Cable America specializes in ultra-fine, high-performance cable assemblies built for miniaturized medical devices where space, flexibility, and signal integrity leave no margin for error. Their expertise is particularly well-suited to applications such as ultrasound probes, endoscopy systems and catheter-based devices that demand tight routing and low-noise signal transmission.
The company’s designs emphasize fine-wire and micro-coax constructions that maintain electrical performance while withstanding repeated flexing in confined geometries. Hybrid cable configurations support complex signal and power requirements without increasing cable diameter, helping device designers meet aggressive size and performance targets.
To support these precision builds, Proterial integrates cleanroom manufacturing, vertically controlled materials and thorough mechanical and electrical validation before assemblies ship. Operating under an ISO 13485-certified quality system, the company maintains detailed documentation and traceability throughout production.
| Pros | Cons |
|---|---|
| ✅ Deep expertise in fine-wire and micro-coax technologies for miniaturized medical devices. | ❌ Higher engineering involvement can increase upfront development effort. |
| ✅ Vertical integration allows tighter control over materials and process consistency. | |
| ✅ Experience supporting complex assemblies used in minimally invasive and precision-driven systems. |
3. Smiths Interconnect
Smiths Interconnect solves complex medical connectivity challenges where signal integrity, ergonomics and evolving interface requirements intersect. The company is best known for advanced interconnect engineering that supports sophisticated medical systems where electrical performance and usability are tightly linked.
Their medical cable assemblies are engineered to address issues like electromagnetic interference and radio-frequency interference (EMI/RFI), miniaturization and repeated connection cycles. Configurable connector solutions, including ergonomic quick-disconnect designs such as the HyperGrip® series, help improve reliability and serviceability.
These technical capabilities are reinforced through the use of biocompatible materials, support for multiple sterilization methods, including steam autoclave, ethylene oxide (EtO) and plasma.
Backed by ISO 13485 quality systems, FDA-registered manufacturing and detailed traceability, Smiths Interconnect supports both custom and standard assemblies at scale while helping OEMs adapt to changing medical interface requirements over time.
| Pros | Cons |
|---|---|
| ✅ Advanced connector designs focused on ergonomics and quick disconnect functionality. | ❌ Highly specialized assemblies may not suit basic cable needs. |
| ✅ Strong EMI/RFI mitigation for sensitive imaging and monitoring systems. | |
| ✅ Micro-coax and hybrid solutions for space-constrained medical devices. |
4. Network Accessories Inc.
NAI Group is built for medical original equipment manufacturers (OEMs) that prioritize process discipline, repeatability and long-term production stability over short-term speed. The company’s strength lies in supporting regulated medical device programs that demand consistent output and controlled change management across the full product lifecycle.
Medical cable assemblies produced by NAI support both reusable and disposable applications. Cleanroom manufacturing and tightly controlled processes help ensure consistency for programs where contamination control and repeatability are critical.
A defining feature of NAI’s approach is its 100% in-process inspection culture, reinforced by validation frameworks such as Production Part Approval Process (PPAP) and Installation Qualification, Operational Qualification and Performance Qualification (IQ/OQ/PQ).
With multiple manufacturing plants and global production capacity, NAI supports low-volume custom engineering as well as sustained high-volume production. This makes them a strong fit for long-life medical programs with evolving demand and delivery requirements.
| Pros | Cons |
|---|---|
| ✅ Strong process discipline supported by 100% inspection during production. | ❌ Not optimized for low-complexity cable builds where speed outweighs process depth. |
| ✅ Readiness for PPAP and IQ/OQ/PQ requirements in regulated programs. | |
| ✅ Experience supporting long-life medical programs with sustained production over many years. |
5. Helukabel
Helukabel differentiates itself through one of the industry’s broadest portfolios of medical-grade cables, backed by global production and inventory depth.
The company is a practical choice for medical device manufacturers that need reliable, readily available cable solutions across a wide range of applications, from diagnostic imaging and surgical equipment to patient monitoring and laboratory systems.
Rather than focusing solely on highly specialized builds, Helukabel’s strength lies in providing proven cable designs that perform consistently across many device types. Their medical cable assemblies are engineered for reliability, electromagnetic compatibility and long service life, with constructions that withstand repeated disinfection and high-temperature sterilisation in clinical environments.
With production sites in multiple countries and an extensive stock of cable types and connectors, Helukabel can support both prototype needs and scaled production. They’re well-suited for programs that value availability, consistency and global delivery.
| Pros | Cons |
|---|---|
| ✅ Exceptionally wide range of medical cable types, including signal, control and spiral options. | ❌ Some medically relevant tests or certifications may vary by region or site. |
| ✅ Global inventory and multiple production sites support responsive delivery. | |
| ✅ Offers autoclavable cables suitable for regular disinfection and sterilisation in clinical environments. |
6. Croax d.o.o.
Croax brings decades of radio frequency (RF) and data transmission expertise into medical cable assemblies where signal accuracy is mission-critical. With more than 30 years of experience in RF and data solutions, the company is a good choice for medical devices used in applications where low noise and stable signal performance directly affect outcomes.
Their medical cable assemblies are engineered with shielded conductors and carefully selected low-noise materials to preserve signal integrity in electrically sensitive environments. These designs support both reusable and single-use clinical applications, helping device manufacturers meet biocompatibility and sterilisation expectations without compromising electrical performance.
Croax reinforces this focus on signal reliability through rigorous electrical, mechanical, and environmental testing before delivery. Operating under an ISO 13485 quality management framework, the company maintains full documentation and traceability from material selection through finished assembly.
Flexible production and delivery from the European Union make Croax a strong partner for OEMs seeking custom, high-precision assemblies with dependable turnaround and regional supply advantages.
| Pros | Cons |
|---|---|
| ✅ Deep RF and data connectivity expertise enhances signal integrity in medical devices. | ❌ Smaller company size might limit large-scale production capacity. |
| ✅ Customisation expertise supports complex device routing and interface needs. | |
| ✅ Flexible EU delivery reduces logistics complexity for regional customers. |
7. Y. C. Cable
Y.C. Cable supports medical original equipment manufacturers (OEMs) that need rapid prototyping today and scalable production tomorrow, backed by a global manufacturing footprint. With facilities across the United States, Asia and Taiwan, the company is well-positioned to help medical device teams move quickly from early builds to sustained volume without switching suppliers or requalifying processes.
Their medical cable assemblies and wire harnesses are designed using biocompatible materials that withstand repeated sterilisation and cleaning while maintaining signal integrity. This balance of durability and electrical performance supports both development-stage and production-ready medical devices.
Y.C. Cable reinforces this speed-to-scale model through thorough electrical and mechanical testing, along with full traceability and documentation from material sourcing through final assembly. Operating under ISO 13485 and related industry standards, the company combines responsive engineering support with global capacity, making it a strong fit for OEMs managing ever-changing specifications, regional production needs and growing demand.
| Pros | Cons |
|---|---|
| ✅ Experience spanning over four decades serving precision medical applications. | ❌ Custom builds may require upfront specification alignment. |
| ✅ Global manufacturing footprint supports both fast prototyping and volume production. | |
| ✅ Offers quick-turn prototyping alongside volume runs. |
8. Custom Wire Industries
Custom Wire Industries works well for medical programs with long lifecycles and legacy design constraints. The company’s strength lies in supporting medical devices that require ongoing updates, incremental revisions or compatibility with established designs rather than frequent full redesigns.
Engineers at Custom Wire Industries work closely with customers from concept through production, helping adapt cable assemblies and lead wires as requirements change over time. This collaborative approach is especially valuable for applications where consistency and backward compatibility matter as much as innovation.
Manufacturing is supported by quality control testing throughout production to catch issues early and maintain dependable performance. Operating under Underwriters Laboratories (UL) and Canadian Standards Association (CSA) certification, with full documentation and traceability maintained from initial drawings through final delivery, the company’s Wisconsin-based facilities support both prototype and production builds with steady turnaround and hands-on technical oversight.
| Pros | Cons |
|---|---|
| ✅ Strong fit for legacy medical devices and revision-driven programs. | ❌ Less emphasis on advanced miniaturization or high-density interconnect trends. |
| ✅ Domestic manufacturing can simplify communication and time zone coordination. | |
| ✅ Well-suited for programs that require frequent small changes over time rather than fixed designs. |
9. Orantech
Orantech stands out for hands-on engineering collaboration, helping medical device teams refine cable designs quickly and clearly during development. The company is a good fit for early-stage and revision-driven programs where close technical communication and fast iteration are essential to keeping projects on schedule.
Their engineers work directly with customers to translate functional requirements into build-ready assemblies, using interactive computer-aided design (CAD) support and revision-friendly workflows. Cable designs use materials and construction methods that withstand repeated handling and exposure to common clinical disinfectants.
As designs mature, Orantech reinforces reliability through electrical and mechanical testing performed during production. Full traceability and documentation are maintained from material lot through final inspection, supporting regulatory submissions and quality audits. With manufacturing capabilities that scale from prototypes to higher-volume runs, Orantech provides a smooth transition from development to production without losing engineering continuity.
| Pros | Cons |
|---|---|
| ✅ Provides interactive CAD support for clearer design communication early in the process. | ❌ Custom focus means fewer off-the-shelf configurations. |
| ✅ Strong hands-on engineering collaboration during early development. | |
| ✅ Known for responsiveness and technical collaboration during development phases. |
10. Wiringo
Wiringo appeals to medical device teams that value speed, sourcing flexibility and fast feasibility feedback when timelines are tight. The company is an excellent choice for projects that require quick decisions, responsive communication, and the ability to adapt as specifications evolve during development or ramp-up.
A notable strength is Wiringo’s rapid quoting process and early technical input, which helps teams refine designs before committing to production. Their ability to source specialized medical-grade components on demand supports both simple and complex cable assemblies.
Reliability is reinforced through comprehensive electrical and mechanical testing performed before delivery, along with full traceability and documentation maintained throughout production. With flexible manufacturing capacity that supports prototypes through higher-volume builds, Wiringo provides a responsive option for regulated medical programs that need to move quickly without sacrificing quality control.
| Pros | Cons |
|---|---|
| ✅ Rapid quoting and early feasibility feedback support fast decision-making. | ❌ Custom assemblies may require detailed upfront specification work. |
| ✅ Flexible build capabilities support both simple and complex harnesses. | |
| ✅ Ability to source specialized medical-grade components on demand. |
What to Look for in Top Medical Cable Assembly Manufacturers
Regulatory-Grade Quality System
Top manufacturers adhere to stringent standards like ISO 13485 and IEC/UL 60601, even for components not requiring full regulatory submission.
These certifications ensure the cables meet safety and performance criteria, particularly for patient contact and critical clinical applications. A proven track record of passing regulatory evaluations along with documented risk and change control processes demonstrates the manufacturer’s commitment to maintaining high-quality, compliant products.
Biocompatible, Sterilization-Ready Designs
Not every medical cable requires biocompatibility or sterilization, but for manufacturers producing cables for patient contact or reuse in clinical environments, these attributes are essential. Top manufacturers use medical-grade, biocompatible materials that resist body fluids and clinical chemicals.
Their cables and overmolds are designed to withstand repeated sterilization cycles such as autoclave, EtO, and gamma sterilization without cracking, delaminating or losing performance. These design choices ensure long-term reliability in demanding healthcare environments.
Robust Performance and Test Engineering
For cables used in critical medical applications, robust performance testing is non-negotiable.
Manufacturers must conduct in-house mechanical, electrical and environmental testing such as flex, pull, Hi-Pot, insulation resistance and EMC tests following IEC 60601 and application-specific limits.
While basic continuity checks may be sufficient for certain cables, the best manufacturers provide detailed validation and lot-level test reports, ensuring long-term reliability and consistent performance in clinical use.
Traceability, Documentation and Engineering Support
Comprehensive documentation is essential for medical device manufacturers, especially those seeking regulatory approvals.
Top manufacturers offer full component-level traceability including lot codes, BOMs and process records along with detailed Device Master Records (DMR) and Device History Records (DHR) for each assembly.
Additionally, strong engineering support including design-for-manufacturability feedback and assistance with regulatory documentation ensures smooth collaboration and helps mitigate risk throughout the product development lifecycle.
Reliable, Compliant Manufacturing Capacity
While not all medical cables need to meet the same requirements, the top manufacturers have proven capabilities to handle both prototypes and high-volume production runs. These manufacturers secure medical-grade connectors, cables and overmolds with options for second sources to reduce supply chain risks.
Their manufacturing capacity is consistently reliable, ensuring compliance and maintaining consistent quality from initial product development to mass production, making them ideal for regulated medical device programs.
Back to Top: Top Medical Cable Assembly Manufacturers


